The Latest Consumer Intelligence
Verified.Org: Protect & Verify Everything Important
Shop Safely with Brands
Although a lot of work has been done to remove counterfeit Apple products from shelves and online stores, fake Apple watches are still easy to come by.
Jesse Sumrak
Cameron Craig
Nicolle Monico
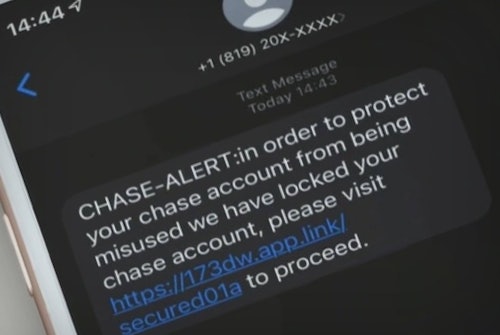
If you've received a locked debit card text message from Citibank, it's likely a scam. Don't click on the link and delete the text message.
Cameron Craig
Verified.org
Beware of buyers on Craigslist who only want to pay for your item with a cashier's check, then overpay you for your items—they're trying to steal money from you.
Verified.org
Verified.org
Verified.org
Verified.org
Bridget Clerkin
Over 1.4 million Americans reported being victims of identity theft in 2021. With the rapid rise of identity theft, it is now more likely that you will become a victim of identity theft than to have your car stolen.
Verified.org
Nicolle Monico
Learn why the Rocket Nail Fuel System is a favorite among those looking to repair dry, brittle, and severely damaged nails—in short, it works.
Tina Chang
Do you need to maintain your car but are cash-strapped? A personal loan is one way to help deal with financial burdens, but does it apply when trying to repair a vehicle?
Verified.org
Pete Ottery
Subscribe: Safety Tips Email